- PhD Tutorial 2017
- BioPPN 2016
- Beijing 2015
- BioPPN 2015
- BioPPN 2014
- Petri Nets 2014
- BioPPN 2013
- Petri Nets 2013
- BioPPN 2012
- ICSB 2011
- BioPPN 2011
- AWPN 2010
- ISS - BioPN 2010
- BioPPN 2010
- Petri Nets 2009
- APBC 2009
- ISMB 2008
- BioSysBio 2008
data structures and software dependability
computer science department
brandenburg university of technology cottbus - senftenberg
An introduction to biomodel engineering, illustrated for signalling pathway
Tutorial at APBC 2009, Beijing, China, 13 January 2009
Rainer Breitling1, David Gilbert2, Monika Heiner3, Robin Donaldson4
(1) Groningen Bioinformatics Centre, University of Groningen, Groningen, Netherlands
(2) School of Information Systems, Computing and Mathematics, Brunel University, Uxbridge, Middlesex UB8 3PH, UK
(3) Computer Science Department, Brandenburg University of Technology, Cottbus, Germany
(4) Department of Computing Science, University of Glasgow, Glasgow, UK
Abstract
Quantitative models of biochemical networks are a central component of modern systems biology. Building and managing these complex models is a major challenge that can benefit from the application of formal methods adopted from theoretical computing science. In this tutorial we provide a general introduction to the field of formal modeling, which emphasizes the intuitive biochemical basis of the modeling process, but is also accessible for an audience with a background in computing science and/or model engineering.
Tutorial level: Introductory
Prior knowledge required: Suitable for biochemists with an understanding of biochemical networks, and enzyme kinetics, as well as for computer scientists and engineers familiar with basic modelling approaches.
Synopsis
Quantitative models of biochemical networks (signal transduction cascades, metabolic pathways, gene regulatory circuits) are a central component of modern systems biology. Building and managing these complex models is a major challenge that can benefit from the application of formal methods adopted from theoretical computing science. In this tutorial we provide a general introduction to the field of formal modelling, which emphasizes the intuitive biochemical basis of the modelling process, but is also accessible for an audience with a background in computing science and/or model engineering.
When building an ODE model, model complexity can rapidly increase to a level that is difficult to manipulate. Computational tools that allow the modular construction and visualization of ODE models would be very helpful. In addition, one usually faces a number of non-trivial design choices during model building. Even for a relatively simple model there may be many ways to describe its dynamic behaviour.
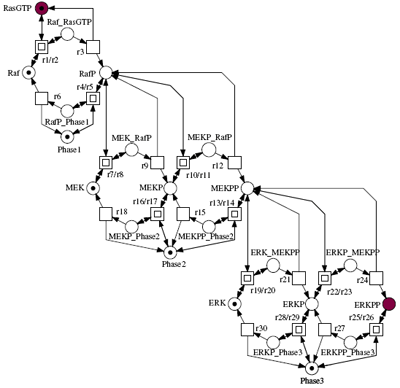
In this tutorial we show how signal transduction cascades can be modelled in a modular fashion, using both a qualitative approach Qualitative Petri nets, and quantitative approaches Continuous Petri nets and Ordinary Differential Equations. We review the major elementary building blocks of a cellular signalling model, discuss which critical design decisions have to be made during model building, and present a number of novel computational tools that can help to explore alternative modular models in an easy and intuitive manner. These tools, which are based on Petri net theory, offer convenient ways of composing hierarchical ODE models, and permit a qualitative analysis of their behaviour.
We will illustrate our approach using a generic model of a signalling cascade, and then relate this to existing models of the MAPK pathway, e.g. Levchenko, Brown and Schoerbel. We will show how the dynamic behaviour of such a pathway is related to its modular structure, and explore the use of stochastic versus deterministic techniques to generate behaviour traces. We will also introduce the use of temporal logic model checking of the pathway to characterise behavioural properties, using software recently developed at the University of Glasgow.
The ultimate aim is to introduce a general approach that provides the foundations for a structured formal engineering of large-scale models of biochemical networks.
We will illustrate the construction of the models in Ordinary Differential Equations using the BioNessie workbench from the University of Glasgow. This SBML compatible tool permits model construction of the models with a biochemistry-oriented interface, parameter scanning, fitting, and model checking using linear temporal logic. We will also illustrate the use of MatLab and the SimBio toolbox for the construction of models, which permits the use of the powerful programming and analytical facilities of MatLab. Petri net approaches will be done using the Snoopy and Charlie tools, both from the Brandenburg University of Technology Cottbus. Snoopy supports qualitative as well as quantitative Petri nets, including continuous and stochastic Petri nets. Snoopy's export feature permits interfacing to various analysis tools devoted to standard Petri net theory, as well as a variety of model checkers. There is also an export allowing access to other tools such as BioNessie, MatLab and the Glasgow model checker permitting more detailed evaluations of continuous and stochastic Petri nets in addition to the standard algorithms of ODE solvers provided by Snoopy.
References
- Rainer Breitling, David Gilbert, Monika Heiner, Richard Orton (2008). A structured approach for the engineering of biochemical network models, illustrated for signalling pathways. Briefings Bioinformatics September 2008; 9: 404 - 421 [preprint]
- Monika Heiner, David Gilbert, and Robin Donaldson (2008), Petri Nets for Systems and Synthetic Biology. In M Bernardo, P Degano, and G Zavattaro (Eds.): SFM 2008, Springer LNCS 5016, pp. 215-264, 2008.
- Xuan Liu, Jipu Jiang, Oluwafemi Ajayi, Xu Gu, David Gilbert, Richard Sinnott (2008). BioNessieG - A Grid Enabled Biochemical Networks Simulation Environment. Accepted for HealthGrid 2008. [preprint]
- David Gilbert, Monika Heiner and Sebastian Lehrack (2007). A Unifying Framework for Modelling and Analysing Biochemical Pathways Using Petri Nets In proceedings CMSB 2007 (Computational Methods in Systems Biology), Editors: M.Calder and S.Gilmore, Springer LNCS/LNBI 4695, pp. 200-216.
- David Gilbert and Monika Heiner, (2006). From Petri Nets to Differential Equations - an Integrative Approach for Biochemical Network Analysis, 27th International Conference on Application and Theory of Petri Nets and other models of concurrency (ATPN06), Turku, Finland, June 26-30, 2006. Proceedings; Editors: Susanna Donatelli, P. S. Thiagarajan; LNCS 4024 / 2006, pp. 181-200, ISBN: 3-540-34699-6 doi:10.1007/11767589
- David Gilbert, Hendrik Fuß, Xu Gu, Richard Orton, Steve Robinson, Vladislav Vyshemirsky, Mary Jo Kurth, C. Stephen Downes and Werner Dubitzky. (2006) Computational methodologies for modelling, analysis and simulation of signalling networks, Briefings in Bioinformatics 2006 7(4):339-353; doi:10.1093/bib/bbl043 Special Issue: Computational Methodologies for Systems Biology. [Abstract], [Full Text], [PDF]
Software
- Snoopy and Charlie
- BioNessie
- MC2
- gillespie2
- MatLab and SimBiology
Models
- Illustrative synthetic models (Petri nets & SBML)
- Levchenko (Petri nets)
- Published models in SBML, including levchenko.xml and kholodenko.xml
Slides
- Part I Biology
- Part II Petri nets
- Part III Biological applications
- Part IV Model checking
- BioNessie
Tutorial URL: http://people.brunel.ac.uk/~csstdrg/workshops/APBC2009/
News
Jobs
The School of Information Systems, Computing and Mathematics at Brunel University in London, UK is strengthening its activities in Bioinformatics and Systems Biology, as well as establishing new activities in Synthetic Biology.
There are several open positions for talented and able postdoctoral researchers and PhD students in the area of Computational Systems and Synthetic Biology at Brunel.
For more details, see David Gilbert's website.
The Groningen Bioinformatics Centre at the University of Groningen, The Netherlands, is looking for creative bioinformaticians with an interest in Systems Biology, Metabolomics, Proteomics, Quantitative Genetics, Network Reconstruction, Dynamic Modelling... to expand its young and successful team.
Several PhD and Postdoc positions are available immediately. For more information on our recent work, have a look at Rainer Breitling's website. There is also a partial list of GBiC vacancies at the GBiC homepage.
If you are interested in a career in Groningen, please try to talk to Rainer.
The International Max Planck Research School for Analysis, Design and Optimization in Chemical and Biochemical Process Engineering in Magdeburg/Germany (IMPRS), offers young researchers a stimulating and challenging atmosphere for education and research in a highly international and interdisciplinary environment. For open positions (PhD students and postdoctoral researchers) see Monika Heiner's website and of her IMPRS cooperation partner Wolfgang Marwan.
Sponsors
The work presented in this tutorial is partially funded under the 6th Framework Programme by the European Commission (Integrated biomedical information for better health), within the context of the SIMAP project.


